Technology
8 min read
Digital Therapeutics: When Mobile Apps Become the Medicine


Digital therapeutics represent an emerging field of healthcare that uses evidence-based mobile medical apps to prevent, manage, and treat medical conditions. Beyond wellness apps that promote healthy behaviors, digital therapeutics are regulated medical devices that require clinical validation through rigorous studies, just like any new pharmaceutical drug.
With skyrocketing rates of chronic diseases worldwide, the healthcare system struggles to provide accessible and affordable solutions at scale. Digital therapeutics offer a promising new model - delivering therapy anytime, anywhere via smartphones and tablets. As software-based treatments, they can scale cost-effectively while maintaining personalized care. This is where a healthcare app development company can play a crucial role, ensuring that these applications are designed with both functionality and user experience in mind.

Digital therapeutics, or "DTx", are a distinct category of digital health solutions. The Digital Therapeutics Alliance defines them as:
“Evidence-based therapeutic interventions driven by high-quality software programs to prevent, manage, or treat a medical disorder or disease. They are validated through clinically evaluated, randomized controlled trials to support product claims on impacting health outcomes.”
Like traditional medicines, DTx:
But unlike traditional pharmaceuticals, DTx:
Chronic conditions like diabetes, hypertension, and asthma afflict over half the global population - and the rates keep rising.
These long-lasting diseases strain healthcare systems and lower quality of life, requiring ongoing medical care and self-management. Medication and lifestyle changes help control them, but many patients still suffer complications.
However, these prevalent conditions also present an opportunity for disruptive health technologies, such as digital therapeutics, that foster prevention and simplify disease management.
Unfortunately, the traditional healthcare model has not adapted well to the rising burden of chronic illnesses. It still focuses primarily on acute, episodic care.
As a result, many healthcare systems struggle to provide consistent, individualized support for lifelong disease management. Patients feel overwhelmed navigating a complex system not designed for their needs.
Key shortcomings include:
These systemic gaps contribute to suboptimal health outcomes, reduced quality of life, and higher costs from complications.
Digital therapeutics present a timely solution that helps address many current healthcare system shortcomings.
As software-based treatments, DTx can extend care beyond traditional settings directly to where patients live. By digitizing elements of therapy, they facilitate access, coordination, patient empowerment, and cost savings.
Delivered via mobile devices, DTx is conveniently accessible at any time, helping increase adherence to treatment plans.
DTx seamlessly integrates information across providers as data-driven solutions to enable holistic care.
DTx promotes self-care through personalized feedback and digital coaching. Gamification elements boost motivation.
Automated remote monitoring reduces unnecessary healthcare utilization. Software scalability also lowers costs.
While formal digital therapeutics, as currently defined, are relatively new, connected “digital pills” laid the conceptual foundation years ago.
Approved by the FDA in 2017, Abilify MyCite became the first smart pill to track medication ingestion digitally. Embedded with an ingestible sensor, the antipsychotic drug helps confirm that patients with mental illness take their medication as prescribed.
The sensor communicates with a wearable patch to detect medication intake. This data can then be shared with caregivers through a smartphone app.
By digitizing a key therapy component - in this case, confirming medication adherence - smart pills foreshadowed the potential of software-based treatments.
They represented an early precursor to today’s multi-component digital therapeutics focused wholly on digital delivery.
Digital health encompasses various software-based tools, such as health education apps, telehealth platforms, wearable fitness trackers, and more. But digital therapeutics stand out as a unique subsegment.
It’s important to distinguish DTx from the recent explosion of wellness apps and health trackers. Popular apps like Headspace (meditation) and MyFitnessPal (diet tracker) help consumers adopt healthy behaviors.
But unlike DTx, wellness apps:
Conversely, digital therapeutics:
This clinical credibility gives DTx special status with healthcare stakeholders.
Digital health is also empowered by telemedicine platforms enabling virtual doctor consultations. But telemedicine and DTx have different roles:
Telemedicine expands access to physician care via online video visits. However, consultations are periodic.
Digital therapeutics deliver the actual therapeutic interventions digitally through software. Treatments can be accessed anytime, as often as needed.
So telemedicine replaces the in-person visit, while digital therapeutics replace or augment in-person therapy administered manually.
Telemedicine and digital therapeutics can provide comprehensive virtual care - consultations and treatment.
Like pharmaceutical products, digital therapeutics must demonstrate safety, efficacy, and quality through rigorous, evidence-based clinical trials.
In the U.S., the Food and Drug Administration (FDA) assesses whether DTx products meet the definition of a medical device and classify them into one of three regulatory categories accordingly:
Class I: Low-risk medical devices like Band-Aid bandages require basic quality testing and manufacturing controls.
Class II: Medium-risk products like powered wheelchairs involve more stringent standards and post-market surveillance.
Class III: Highest-risk devices like pacemakers must submit a premarket approval application with clinical data.
Digital therapeutics typically fall under Class II, which requires submitting 510(k) premarket notifications to the FDA demonstrating “substantial equivalence” to an already approved device, or Class I.
Digital therapeutics undergo extensive clinical testing and evaluation analogous to the drug development process:
Meeting established clinical guidelines and endpoints, digital therapeutics accumulate evidence like drugs to support their therapeutic claims.
Pharmaceutical companies recognize mobile apps could boost patient compliance and outcomes when combined with their drugs.
Under this “drug + software” model, the software component handles key therapy aspects like medication reminders, symptom tracking logs, or visual coordination tasks that integrate with the drug’s mechanisms.
For example, Akili's EndeavorRx DTx improves cognitive functioning in kids with ADHD by leveraging sensory and motor game mechanics. This neurologic stimulus complements the pharmacological activity of stimulant medications used to treat ADHD.
Such complementary digital programs can enhance adherence, treatment effectiveness, and health system efficiency.
When designed thoughtfully, evaluated rigorously, and prescribed appropriately, digital therapeutics can:
The global digital therapeutics market is poised for massive growth, driven by an aging population and rising chronic disease burden.
Market Size Projections
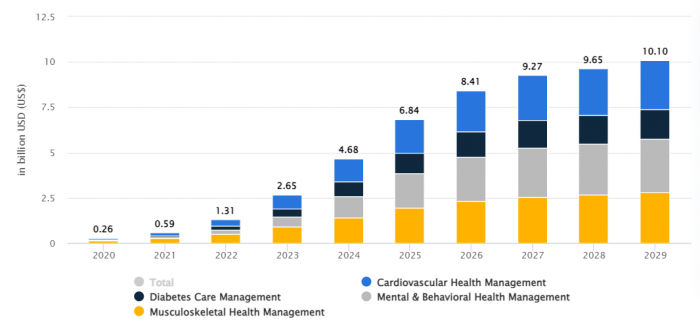
-$8.4 billion by 2026
-$10.1 billion by 2029
Key Growth Drivers
As outcomes data mounts and awareness grows, digital therapeutics adoption will accelerate across healthcare systems globally.
Digital therapeutics represent the vanguard of a broader “software as medicine” movement brewing in healthcare.
Currently, doctors prescribe drugs and medical devices. Soon, software-based treatments could also be included on prescription pads, along with other traditional interventions.
This radical shift envisions clinicians one day “prescribing” mobile health apps with the same ease and legitimacy as pharmaceutical drugs.
Prescribed digital therapeutics could manage chronic conditions and address mild acute illnesses that don’t require in-person emergency care or elaborate drug regimens.
Ultimately, digital therapeutics expand the toolbox for delivering accessible, scalable treatments - paving the way for the coming age of “prescribable software”.
In summary, digital therapeutics herald an exciting evolution in healthcare. Backed by gold-standard evidence, they transform mobile devices into regulated medical interventions.
ADigitaltherapeutics boosts access, coordination, patient engagement, and value by addressing systemic gaps. Early applications already improve outcomes for many chronic conditions while tackling medication compliance and mental health.
As research expands, costs decline, and health systems integrate them, prescribed DTx will soon become standard components of care regimens. Just as digital disruptions revolutionized other industries, innovative software-based treatments aim to democratize medicine, proving that proper health need not come just from a pill.
Be the first to post comment!